Submission Instruction
Section 1 Publishing Scope
Section 2 Requirement for Manuscripts
Section 3 Fees and Renumeration
Section 4 Method of Submission
Section 1 Publishing Scope
Journal of New Medicine aims at the integration of basic research and clinical practice of medicine, reporting new theories, new knowledge and new diagnosis and treatment techniques of various disciplines of clinical medicine. Articles such as Editorial, Review, Original Research and Case Research are welcomed. The journal mainly accepts manuscripts in basic or clinical medicine (involving mechanism, diagnosis, and treatment) and does not accept manuscripts in nursing studies, education, and pure meta-analysis.
Section 2 Requirement for Manuscripts
1 General Requirement: Manuscripts should be cutting-edge, scientific and practical, with clear argumentation, authentic material, concise writing, clear structure, accurate data. Proper statistical processing should be adopted if necessary. The content should be in close combination with clinical practice. The format of writing should comply with the requirements of the journal.
2 Column and Wordcount: Review should be within 6000 words (excluding tables, figures and references). Case Research should be specific analysis of rare or special cases, which shall include the history, diagnosis and treatment process of the case and the date of clinical tests, and the number of words should be less than 5000(excluding tables, figures and references).
3 Typesetting: Manuscripts should be in word (*.doc) or latex (*.tex) format. Please use No.12 character size and 1.5 times spacing. Special format such as superscript should be clearly indicated. Languages other than Chinese and English should be noted clearly. Full text must be carefully proofread. Drug name, dose and data must be double checked.
4 Title: Title should be concise and clear, well reflecting the content of the article. Chinese title should be within 20 Chinese characters. Punctuation or abbreviation should be avoided. English title should be consistent with Chinese title in meaning.
5 Author Information: Author names are arranged in order under the title. If the corresponding author of the manuscript is not the first author, it should be specially noted. Generally, there should be only one corresponding author which is collectively determined. Members of collaboration group should be listed at the end of the text before the references. The phone numbers and email addresses of the first author or corresponding author should be indicated at the end of the text. The authors’ English names should adopt Chinese Pinyin. Beneath the author names should be institutes (specifying department), city (or place), postal code and country. The above information should be both in Chinese and English.
6 Abstract: Articles of all columns should have Chinese and English abstracts. For Original Research, abstract should be within 300 Chinese characters, which includes Objective, Methods, Results and Conclusion. Abstracts should be written in the third person perspective, without figures, tables, references, comments and explanations. Abstracts should be accurate, concise, independent and self-evident. The English abstract should be consistent with the Chinese abstract in content. Please refer to the Requirements for Abstracts of Original Research.
7 Keywords: 2 ~ 5 keywords should be attached after the abstract. Authors should try to use the words from the latest edition of MeSH of the National Library of Medicine (US). Acronyms shall be avoided. For Chinese translation, authors can refer to the Chinese Medical Thesaurus compiled by the Institute of Medical Information, Chinese Academy of Medical Sciences. The new professional terms (free words) that are not included in the thesaurus above can also be used as keywords but should be put at the end. Keywords of traditional Chinese medicine should be selected from the list of Traditional Chinese Medicine Subject Words compiled by the Institute of Traditional Chinese Medicine Information, Chinese Academy of Traditional Chinese Medicine. Manuscripts with English abstracts should be marked with English keywords corresponding to Chinese keywords, and acronyms shall be avoided.
8 Funding Projects: If the manuscript is supported by a fund or belong to a key research project at national, ministerial or provincial level, the funding project should be noted in the footer at the bottom left of the title page, such as “supported by ×× Project/Fund (No ××)”, The approval certificate (copy) should be provided during submission.
9 Terminology: Medical terms shall be selected from those approved by the National Science and Technology Terminology Examination and Approval Committee. Traditional Chinese Medicine (TCM) terms shall be in accordance with Clinical Diagnosis and Treatment Terminology of Traditional Chinese Medicine - Disease, Symptom and Treatment (GB/T 16751.1-1997) Meridian Acupuncture Terms shall be in accordance with Meridian Points (GB/T 16751.2-1997) and Meridian Points of Ear (GB/T 16751.3-1997). Authors can refer to Chinese and English Glossary of Common Medical Terms on this website.
10 Acronyms: Generally, acronyms should be used as less as possible and there should be no more than 5 acronyms throughout an article. For medical terms of four or less Chinese characters, acronym should be avoided. For terms of more than 4 Chinese characters for which acronyms are necessary, the Chinese and English full names should be indicated for the first time in the manuscript. E.g., “动脉血氧分压 (partial pressure of oxygen in arterial blood, PaO2)”. English acronyms shall not have breaking-line. Chinese abbreviations shall be put in parenthesis after the full name. E.g., “再生障碍性贫血(再障)”. The author can refer to the Chinese and English Glossary of Common Medical Terms on this website.
11 Statistical Method
11.1 Statistical Symbols :The journal implements the relevant provisions of Statistical Terms and Symbols (GB3358-1982). All statistical symbols are in italics: ① the mean ± standard deviation is in ( ±s), English lowercase and italic; ② t-test is in English lowercase and italic; ③ F-test is in English capital and italic; ④ χ2 is in Greek lowercase and italic; ⑤ correlation coefficient is in r, English lowercase and italic; ⑥ Probability is in P, English capital and italic.
11.2 Research Design: The name and main methods of the research design should be specified, such as Survey Design (whether it is a prospective, retrospective or cross-sectional study), Experimental Design (including the specific design types, such as self-paired design, group design, crossover design, factorial design, orthogonal design, etc.), Clinical Trial Design (including the phase of the clinical trial, random double-blind or random single blind, etc.). The main methods should be summarized in four aspects (repetition, randomization, control, balance). The control of non-experimental factor’s influence shall be elaborated.
11.3 Representation and Description of Data: Normal distribution is expressed by (
±s), English lowercase and italic; ② t-test is in English lowercase and italic; ③ F-test is in English capital and italic; ④ χ2 is in Greek lowercase and italic; ⑤ correlation coefficient is in r, English lowercase and italic; ⑥ Probability is in P, English capital and italic.
11.2 Research Design: The name and main methods of the research design should be specified, such as Survey Design (whether it is a prospective, retrospective or cross-sectional study), Experimental Design (including the specific design types, such as self-paired design, group design, crossover design, factorial design, orthogonal design, etc.), Clinical Trial Design (including the phase of the clinical trial, random double-blind or random single blind, etc.). The main methods should be summarized in four aspects (repetition, randomization, control, balance). The control of non-experimental factor’s influence shall be elaborated.
11.3 Representation and Description of Data: Normal distribution is expressed by (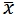 ±s). Skew distribution is expressed by full range (median). For tables, the vertical and horizontal headings should be well arranged, and the meaning of data should be clearly expressed. For figures, the type of statistical chart should match the nature of data, and the scaling method on the number axis should conform to mathematical principles. For relative numbers, the denominator should not be less than 20.
11.4 Selection of Statistical Methods: For quantitative variable, appropriate statistical methods should be selected according to different types of design, conditions and analysis purpose. Do not simply apply the t-test and analysis of variance. For qualitative variables, appropriate statistical analysis methods should be selected according to the design type used, the conditions of the nature and frequency of qualitative variables and the purpose of analysis. Do not simply apply χ2 test and rank-sum test. For regression analysis, appropriate regression types should be selected according to professional knowledge and scatter diagram. Do not always apply simple linear regression analysis. For regression analysis with repeated experimental data, the processing should not be simplified. For multi-factor and multi-index data, it is necessary to make a comprehensive and reasonable explanation and evaluation on the interaction between factors and the internal relationship among indexes on the basis of univariate analysis using multivariate statistical analysis methods.
11.5 Interpretation and Expression of Statistical Results: When P<0.05 or P<0.01, it shall be concluded that the difference between the contrastive groups is statistically significant, rather than that there is significant (or very significant) difference between contrastive groups. The specific name of statistical analysis method used shall be indicated (such as t-test of group design data, variance analysis of two-factor factorial design data, etc.).
12 Tables and Figures: Tables and figures should be numbered consecutively according to the order in which they appear in the text and should follow the content which they are referring to. Each table (figure) shall be preceded by a heading and followed by notes below the table (figure), in which all uncommon acronyms used in the table (figure) shall be indicated. The journal adopts three-line style for tables (top-line, heading-line and bottom-line). It is required that the effective digits of the same index are consistent, which is generally the same as 1/3 of the standard deviation. Laser printing drawings shall be provided for computer draft. It is recommended that pictures are scanned and have a good clarity and contrast. Please guarantee symbols (including arrows) on scanned pictures are correctly marked except for photographs. Line drawings are also recommended to be scanned or processed by computer. If human portrait is used, written consent should be obtained or the figure shall be pixeled. Body photos should have scale marks. Pathological photos should be indicated with staining method and magnification. If there is any quotation from other publications in the tables (figures), the credit shall be given properly. JPG format is recommended for electronic pictures. Please refer to the Requirement for Tables and Figures on this website.
13 Drug Names: the TCM drug names in the manuscript should comply with names in pharmacopoeia or be referred to by general names. English drug names should comply with names in pharmacopoeia or international non-patented drug names. Trade names should not be used in title or main body text. If it is necessary to mention the trade name, its general name should be indicated first, and the main ingredients of a compound should be indicated. Latin names should be indicated for herbal medicines, including family and genus.
14 Numbers: The journal implements Regulations of Numbers in Publications (GB/T 15835-2011). The effective digit shall be determined according to the provisions of Rules for Rounding Off of Numerical Values (GB/T 8170-1987). Please refer to the Chinese version of this website for detailed use of Arabic numerals.
15 Hierarchy: Please refer to the format of each column of this journal or use 1, 1.1, 1.1.1, 1.1.1. for headings and ①, ②, ③ for in-paragraph points.
16 Unit of measurement: Unit of measurement shall comply with the provisions and writing rules of Quantities, Units and Symbols (gb3100 ~ 3102-1993). For specific guidelines, please refer to: Application of Legal Units of Measurement in Medicine (3rd Edition) [M] by Chinese Medical Association. Unit names and unit symbols cannot be confused. According to the Regulations and Supplement on the Use of Blood Pressure Measurement ([1998] No. 126) jointly issued by the State Administration of Quality and Technical Supervision and the Ministry Of Health, millimeter mercury (mmHg) or centimeter water column (cmH2O) can be used as the measurement unit for all pressure measurements involving human and animal bodies, but the conversion coefficient between mmHg or cmH2O and kPa (1 mmHg=0.133 kPa, 1 cmH2O=0.098 kPa) should be indicated when it is used for the first time.
17 References: References should comply with Rules for The Description of Bibliographic References (GB/T 7714-2015). The references should be marked with Arabic numerals in square brackets according to the order in which they appear in the text and shall be listed at the end of the article. Abstracts, internal materials, unpublished materials, personal communications should not be cited as references. References must be read by authors in person. It is recommended to cite literatures in the recent 3 year. Japanese Chinese characters should be written according to Japanese regulations and should not be confused with Chinese standards. The language of literature in references should be indicated if it is not English or Chinese. Generally, the number of references for Original Research should be within 12, and the number of references for Review should be within 30. The format is as follows.
17.1 Authors: All authors must be listed, names separated by commas instead of "和/and". For all authors, surname should come first. Chinese author names are written in accordance with the requirements of Basic Rules of Orthography of Chinese Pinyin (GB/T 16159-1996) and names cannot be abbreviated. Foreign names are abbreviated to the initial letter without dot and a space of 1/4 Chinese character shall be put between names.
17.2 Items: All items for each reference should be completed instead of marking an "同上" or "ibid".
17.3 Format:
Journal
Author. Article Title. Journal Title (Abbreviations for foreign titles as in Index Medicus), year, volume (issue): Start Page - End page. Example:
[References]
[1] 赵云峰,林勇,吴学玲. 早期乳酸清除率对重症肺炎患者预后的评估价值──附42例分析. 新医学,2008,39(11):713-716.
[2] Sharma S, Nemeth E, Chen Y H, et al. Involvement of hepcidin in the anemia of multiple myeloma. Clin Cancer Res, 2008, 14(11): 3262-3267.
Book
Author. Book Title. Edition (omitted for the 1st edition). Place [unknown]: Publisher [unknown], year: Start Page - end page. Example:
[References]
[1] 陆再英,钟南山. 内科学. 7版. 北京:人民卫生出版社,2008:110-116.
[2] McDonald J, Burroughs A, Feagan B. Evidence-based Gastroenterology and Hepatology. 2th ed. London: The Blackwell publishing Ltd,2004:3.
18 Others: For other requirements, please refer to the Detailed Requirement for Manuscript at "Download" section of this website.
Section 3 Fees and Renumeration
1 Procedure: After the manuscript is accepted, the editorial office will send the proofreading file and an APC notice (RMB 500 / page) to the author by mail or email. The APC notice is generally issued one month before publication. The author must make the payment before the deadline or the submission will be withdrawn. The journal does not charge review fee.
2 Method:
Payee: 中山大学附属第三医院(广州)期刊有限责任公司
Bank of Deposit: Industrial and Commercial Bank of China Guangzhou Shipai Branch
Account Number: 3602098609200099938
Transaction can be operated at ATM or the counter of Industrial and Commercial Bank of China. Please send an E-mail to: zssyqkzx@163.com after remittance with the subject of the name of the remitter. Please provide following information in the E-mail: journal title, number of manuscript (if any), name of the first author, name of the remitter, invoice title, mobile phone number, and invoice mailing address (if applicable). The address should be as detailed as possible.
3 Invoice: The title of the invoice should be the full name of the institute or the name of an individual. Please confirm it with your financial department. If the postscript is incomplete or our office cannot verify the payment, the invoice would not be issued. Should the title be incorrect, the invoice would not be re-issued.
4 Renumeration: The journal will renumerate the authors by bank remittance. Please provide the name of the payee (the first author), account number, bank and branch, ID number (for tax declaration) and mobile phone number. The renumeration will be make in the next month from the publishing of article.
Section 4 Method of Submission
1.Submission Method: Please visit www.xinyixue.cn and submit your manuscript at "Submission/Manuscript". The author can check the status of the manuscript online at any time. Authors can make inquiry to the editorial office Three months after submission by the manuscript number. The journal does not accept submission by mail or E-mail. All submission to this journal shall be deemed as acceptance of the journal’s policies.
2.Submission Steps:
Step 1: Click on the icon of "Submission/Manuscript" at the right of the website home page.
Step 2: If it is your first time to submit, please click on "Register". If you have successfully registered beforehand, please skip to step 5.
Step 3: Please fill in your information. Items with “*” are compulsory. Please note:
1) In order to keep in touch with you, contact information should be as complete as possible.
2) Personal information should be authentic and verifiable.
3) The username may not be your real name. You can choose any username that is easy for you to remember as long as it meets the requirement.
4) Please double check the information you fill in before saving and submitting.
Step 4: The system shall prompt a notice "register successful" and you can click on the "OK" button. If there is no such notice prompted to you, the information you filled in may be incomplete or may not meet the system requirement. Please check and fill in again.
Step 5: The system returns to the login session. Please fill in your registered username and password, and then click on the "Login" icon.
Step 6: In the system, you can choose different pages by clicking on titles in the column at the left. Pause the mouse arrow on the corresponding title for a moment, and the system will prompt corresponding instructions. Please click on "Submission" to set up a new manuscript.
Step 7: Click on "Continue" to go on. If it is your first time to submit to the Journal of New Medicine, please download the "Submission Instruction" and read it carefully before continuing.
Step 8: Download the " Copyright Transfer Agreement ". If you receive the Notice of Acceptance, please print the Agreement, fill in the information, have it stamped by your institute, and then submit it through the submission system or E-mail. Then click on "Continue".
Step 9: Please fill in the information required by the system, and the system will guide you to complete your submission.
3.Supporting Documents: In addition to the manuscript, the following documents shall be provided.
1) Recommendation Letter
2) Copyright Transfer Agreement
3) Authors Contribution Form
4) Statement of Competing Interests
5) Certificate of Funding Projects
6) Copy of approval documents of medical ethics or animal rights committee (if applicable)
After publishing, if a prize is rewarded to the article, the authors should inform the editorial office and provide a copy of certificate.
4.Fast Track: The journal provides "fast track" service to major research results funded by national, provincial or ministerial projects. For authors applying for publishing in "fast track", they must provide a written description of the innovation of the article, a "plagiarism testing report" issued by the institute of medical information or libraries at provincial level or above, and recommendation letters from two peer experts with senior professional title. Upon approval, the manuscript will be published as soon as possible.
±s). Skew distribution is expressed by full range (median). For tables, the vertical and horizontal headings should be well arranged, and the meaning of data should be clearly expressed. For figures, the type of statistical chart should match the nature of data, and the scaling method on the number axis should conform to mathematical principles. For relative numbers, the denominator should not be less than 20.
11.4 Selection of Statistical Methods: For quantitative variable, appropriate statistical methods should be selected according to different types of design, conditions and analysis purpose. Do not simply apply the t-test and analysis of variance. For qualitative variables, appropriate statistical analysis methods should be selected according to the design type used, the conditions of the nature and frequency of qualitative variables and the purpose of analysis. Do not simply apply χ2 test and rank-sum test. For regression analysis, appropriate regression types should be selected according to professional knowledge and scatter diagram. Do not always apply simple linear regression analysis. For regression analysis with repeated experimental data, the processing should not be simplified. For multi-factor and multi-index data, it is necessary to make a comprehensive and reasonable explanation and evaluation on the interaction between factors and the internal relationship among indexes on the basis of univariate analysis using multivariate statistical analysis methods.
11.5 Interpretation and Expression of Statistical Results: When P<0.05 or P<0.01, it shall be concluded that the difference between the contrastive groups is statistically significant, rather than that there is significant (or very significant) difference between contrastive groups. The specific name of statistical analysis method used shall be indicated (such as t-test of group design data, variance analysis of two-factor factorial design data, etc.).
12 Tables and Figures: Tables and figures should be numbered consecutively according to the order in which they appear in the text and should follow the content which they are referring to. Each table (figure) shall be preceded by a heading and followed by notes below the table (figure), in which all uncommon acronyms used in the table (figure) shall be indicated. The journal adopts three-line style for tables (top-line, heading-line and bottom-line). It is required that the effective digits of the same index are consistent, which is generally the same as 1/3 of the standard deviation. Laser printing drawings shall be provided for computer draft. It is recommended that pictures are scanned and have a good clarity and contrast. Please guarantee symbols (including arrows) on scanned pictures are correctly marked except for photographs. Line drawings are also recommended to be scanned or processed by computer. If human portrait is used, written consent should be obtained or the figure shall be pixeled. Body photos should have scale marks. Pathological photos should be indicated with staining method and magnification. If there is any quotation from other publications in the tables (figures), the credit shall be given properly. JPG format is recommended for electronic pictures. Please refer to the Requirement for Tables and Figures on this website.
13 Drug Names: the TCM drug names in the manuscript should comply with names in pharmacopoeia or be referred to by general names. English drug names should comply with names in pharmacopoeia or international non-patented drug names. Trade names should not be used in title or main body text. If it is necessary to mention the trade name, its general name should be indicated first, and the main ingredients of a compound should be indicated. Latin names should be indicated for herbal medicines, including family and genus.
14 Numbers: The journal implements Regulations of Numbers in Publications (GB/T 15835-2011). The effective digit shall be determined according to the provisions of Rules for Rounding Off of Numerical Values (GB/T 8170-1987). Please refer to the Chinese version of this website for detailed use of Arabic numerals.
15 Hierarchy: Please refer to the format of each column of this journal or use 1, 1.1, 1.1.1, 1.1.1. for headings and ①, ②, ③ for in-paragraph points.
16 Unit of measurement: Unit of measurement shall comply with the provisions and writing rules of Quantities, Units and Symbols (gb3100 ~ 3102-1993). For specific guidelines, please refer to: Application of Legal Units of Measurement in Medicine (3rd Edition) [M] by Chinese Medical Association. Unit names and unit symbols cannot be confused. According to the Regulations and Supplement on the Use of Blood Pressure Measurement ([1998] No. 126) jointly issued by the State Administration of Quality and Technical Supervision and the Ministry Of Health, millimeter mercury (mmHg) or centimeter water column (cmH2O) can be used as the measurement unit for all pressure measurements involving human and animal bodies, but the conversion coefficient between mmHg or cmH2O and kPa (1 mmHg=0.133 kPa, 1 cmH2O=0.098 kPa) should be indicated when it is used for the first time.
17 References: References should comply with Rules for The Description of Bibliographic References (GB/T 7714-2015). The references should be marked with Arabic numerals in square brackets according to the order in which they appear in the text and shall be listed at the end of the article. Abstracts, internal materials, unpublished materials, personal communications should not be cited as references. References must be read by authors in person. It is recommended to cite literatures in the recent 3 year. Japanese Chinese characters should be written according to Japanese regulations and should not be confused with Chinese standards. The language of literature in references should be indicated if it is not English or Chinese. Generally, the number of references for Original Research should be within 12, and the number of references for Review should be within 30. The format is as follows.
17.1 Authors: All authors must be listed, names separated by commas instead of "和/and". For all authors, surname should come first. Chinese author names are written in accordance with the requirements of Basic Rules of Orthography of Chinese Pinyin (GB/T 16159-1996) and names cannot be abbreviated. Foreign names are abbreviated to the initial letter without dot and a space of 1/4 Chinese character shall be put between names.
17.2 Items: All items for each reference should be completed instead of marking an "同上" or "ibid".
17.3 Format:
Journal
Author. Article Title. Journal Title (Abbreviations for foreign titles as in Index Medicus), year, volume (issue): Start Page - End page. Example:
[References]
[1] 赵云峰,林勇,吴学玲. 早期乳酸清除率对重症肺炎患者预后的评估价值──附42例分析. 新医学,2008,39(11):713-716.
[2] Sharma S, Nemeth E, Chen Y H, et al. Involvement of hepcidin in the anemia of multiple myeloma. Clin Cancer Res, 2008, 14(11): 3262-3267.
Book
Author. Book Title. Edition (omitted for the 1st edition). Place [unknown]: Publisher [unknown], year: Start Page - end page. Example:
[References]
[1] 陆再英,钟南山. 内科学. 7版. 北京:人民卫生出版社,2008:110-116.
[2] McDonald J, Burroughs A, Feagan B. Evidence-based Gastroenterology and Hepatology. 2th ed. London: The Blackwell publishing Ltd,2004:3.
18 Others: For other requirements, please refer to the Detailed Requirement for Manuscript at "Download" section of this website.
Section 3 Fees and Renumeration
1 Procedure: After the manuscript is accepted, the editorial office will send the proofreading file and an APC notice (RMB 500 / page) to the author by mail or email. The APC notice is generally issued one month before publication. The author must make the payment before the deadline or the submission will be withdrawn. The journal does not charge review fee.
2 Method:
Payee: 中山大学附属第三医院(广州)期刊有限责任公司
Bank of Deposit: Industrial and Commercial Bank of China Guangzhou Shipai Branch
Account Number: 3602098609200099938
Transaction can be operated at ATM or the counter of Industrial and Commercial Bank of China. Please send an E-mail to: zssyqkzx@163.com after remittance with the subject of the name of the remitter. Please provide following information in the E-mail: journal title, number of manuscript (if any), name of the first author, name of the remitter, invoice title, mobile phone number, and invoice mailing address (if applicable). The address should be as detailed as possible.
3 Invoice: The title of the invoice should be the full name of the institute or the name of an individual. Please confirm it with your financial department. If the postscript is incomplete or our office cannot verify the payment, the invoice would not be issued. Should the title be incorrect, the invoice would not be re-issued.
4 Renumeration: The journal will renumerate the authors by bank remittance. Please provide the name of the payee (the first author), account number, bank and branch, ID number (for tax declaration) and mobile phone number. The renumeration will be make in the next month from the publishing of article.
Section 4 Method of Submission
1.Submission Method: Please visit www.xinyixue.cn and submit your manuscript at "Submission/Manuscript". The author can check the status of the manuscript online at any time. Authors can make inquiry to the editorial office Three months after submission by the manuscript number. The journal does not accept submission by mail or E-mail. All submission to this journal shall be deemed as acceptance of the journal’s policies.
2.Submission Steps:
Step 1: Click on the icon of "Submission/Manuscript" at the right of the website home page.
Step 2: If it is your first time to submit, please click on "Register". If you have successfully registered beforehand, please skip to step 5.
Step 3: Please fill in your information. Items with “*” are compulsory. Please note:
1) In order to keep in touch with you, contact information should be as complete as possible.
2) Personal information should be authentic and verifiable.
3) The username may not be your real name. You can choose any username that is easy for you to remember as long as it meets the requirement.
4) Please double check the information you fill in before saving and submitting.
Step 4: The system shall prompt a notice "register successful" and you can click on the "OK" button. If there is no such notice prompted to you, the information you filled in may be incomplete or may not meet the system requirement. Please check and fill in again.
Step 5: The system returns to the login session. Please fill in your registered username and password, and then click on the "Login" icon.
Step 6: In the system, you can choose different pages by clicking on titles in the column at the left. Pause the mouse arrow on the corresponding title for a moment, and the system will prompt corresponding instructions. Please click on "Submission" to set up a new manuscript.
Step 7: Click on "Continue" to go on. If it is your first time to submit to the Journal of New Medicine, please download the "Submission Instruction" and read it carefully before continuing.
Step 8: Download the " Copyright Transfer Agreement ". If you receive the Notice of Acceptance, please print the Agreement, fill in the information, have it stamped by your institute, and then submit it through the submission system or E-mail. Then click on "Continue".
Step 9: Please fill in the information required by the system, and the system will guide you to complete your submission.
3.Supporting Documents: In addition to the manuscript, the following documents shall be provided.
1) Recommendation Letter
2) Copyright Transfer Agreement
3) Authors Contribution Form
4) Statement of Competing Interests
5) Certificate of Funding Projects
6) Copy of approval documents of medical ethics or animal rights committee (if applicable)
After publishing, if a prize is rewarded to the article, the authors should inform the editorial office and provide a copy of certificate.
4.Fast Track: The journal provides "fast track" service to major research results funded by national, provincial or ministerial projects. For authors applying for publishing in "fast track", they must provide a written description of the innovation of the article, a "plagiarism testing report" issued by the institute of medical information or libraries at provincial level or above, and recommendation letters from two peer experts with senior professional title. Upon approval, the manuscript will be published as soon as possible.
Pubdate: 2022-08-16
Viewed:
20401